Electronic Laboratory Reporting (ELR) for Communicable Diseases
Electronic Laboratory Reporting (ELR) is the automated reporting of communicable diseases to the Kentucky Department for Public Health (KDPH), in accordance with 902 KAR 2:020 and the Kentucky Reportable Diseases and Conditions. The benefits of ELR are significant for both healthcare providers and public health authorities as it improves timeliness of reports and reduction of manual errors.
By leveraging ELR, healthcare providers and public health authorities can take appropriate actions to limit the spread of diseases and manage impacts effectively. Expansion of ELR improves public health surveillance capabilities.
Two Ways to Submit Laboratory Reports
KHIE offers two options for reporting communicable conditions to KDPH.
Direct Data Entry (DDE)
Direct Data Entry is a secure, web-based reporting applicaton hosted in KHIE's ePartnerViewer platform. It facilitates reporting of laboratory results using KHIE's online web forms. The platform is easily accessible from most web browsers using a secure internet connection. DDE is the KDPH's preferred interim reporting platform. For more information on DDE, click here.
Note: DDE does not meet the MIPS requirements for electronic reporting.
Electronic Submission
Electronic submission is the secure and automatic transmission of laboratory results from Participants through KHIE to KDPH for communicable disease investigation. To ensure the KHIE onboarding process runs efficiently, the KHIE Participant should engage in the following preliminary preparation:
1. Sign the KHIE Participation Agreement and the Reportable Diseases Surveillance Authorization Addendum.
2. Confirm your organization can electronically submit laboratory data either from the laboratory information system (LIS), the electronic health record (EHR), or electronic medical record (EMR).
3. Prepare a list which includes your organization's NPI, CLIA, and location information.
4. Review the KHIE ELR Onboarding Guide.
5. For specific onboarding information, contact KHIELabSupport@ky.gov
The Onboarding Process
The ELR Onboarding guide contains information on all required data elements, formatting of HL7 messages, resources, and best practices for successful onboarding and submission to KHIE and KDPH. This guide serves as a companion to the HL7 Version 2.5.1 Implementation Guide provided by HL7.org.
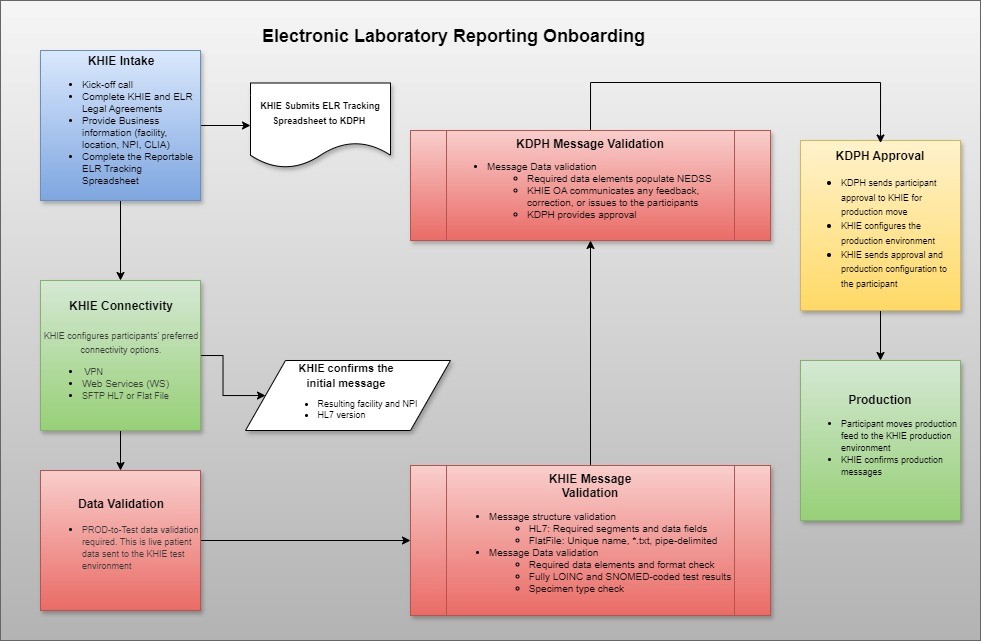
KHIE Intake
- Complete the KHIE legal agreements.
- Complete the Reportable ELR Tracking Spreadsheet.
Connectivity
Options for electronic submission of laboratory results data:
- Virtual Private Network (VPN) - HL7 messaging
- Web Services (WS) - HL7 messaging
- Secure File Transfer Protocol (SFTP) via MoveIt folder - HL7 messaging or pipe delimited (,txt) Flat File
Testing in KHIE's Test Environment
- Generate a test message into KHIE's test environment to check connectivity and message structure.
- When test messages are error-free, send live data to the test environment.
Validation
- Validations are conducted in the KHIE system and in the KDPH system known as National Electronic Disease Surveillance System (NEDSS).
- When KHIE validation is complete, the NEDSS validation will require 10 error-free results from each program area.
- Types of Validations
- Structural Validation
- Review the structure of ELR messages to ensure HL7 standards are met.
- Data Quality Validation
- Assess the data accuracy, completeness, timeliness, and jurisdictional routing.
- Other Validation
- Assess and resolve issues identified by KHIE or KDPH during the validation process.
Production Approval
- Receive approval confirmation email from KDPH.
- KHIE will place Participant into production status.
- Participant will discontinue traditional reporting of Kentucky reportable conditions.
The Kentucky Information Exchange continues to keep pace with requirements set forth by the Department of Health and Human Services (HHS) and the KDPH to ensure that ELR feeds through KHIE meet the standards.
INFLUENCING THE WAY HEALTHCARE IS PLANNED, COORDINATED, AND DELIVERED.
Page Content Updated 05_13_2025