Electronic Case Reporting (eCR)
Electronic Case Reporting is the secure creation and transmission of case reports from an electronic health record (EHR) to public health agencies (PHAs) for disease tracking, case management, and contact tracing. Like traditional reporting, eCR captures critical patient and clinical data regarding demographics, comorbidities, immunizations, medications, and other treatments, but greatly reduces the burden of manual reporting.
KHIE Participants can submit electronic case reports to the Kentucky Department for Public Health (KDPH) through KHIE to meet case reporting requirements, in accordance with 902 KAR 2:020 and the
Table of Reportable Diseases and Conditions in Kentucky.
Leverage KHIE to fulfill state-mandated reporting requirements for communicable diseases and their associated case reports.
Onboard to Submit Case Reports
KHIE offers two options to report notifiable conditions to KDPH.
Direct Data Entry (DDE) Submission
Direct Data Entry is a secure, web-based reporting application hosted in KHIE's ePartnerViewer platform. This platform facilitates case reporting using KHIE's online webforms. The platform is easily accessible from most web browsers using a secure internet connection. Click here to learn more about Direct Data Entry,
Electronic Submission (eCR)
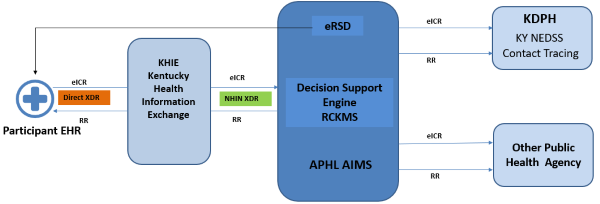
Electronic Case Reporting is the secure transmission of case reports from an electronic health record system that is submitted through KHIE to KDPH for communicable disease investigation. Through Direct Secure Messaging (DSM), KHIE Participants can electronically submit case reports, in real time, directly out of their EMR/EHR system. It makes the process faster and more accurate, timely, and reliable. Electronic case reporting enhances the Electronic Laboratory Reporting (ELR) service KHIE already offers, as it further streamlines the workflow, minimizing the burden of reporting requirements.
To ensure the KHIE onboarding process runs efficiently, the KHIE Participant should engage in the following preliminary preparation:
1. Sign the KHIE Participation Agreement and the eCR addendum.
2. Confirm your EMR/EHR vendor has the capability to electronically submit a case report .
3. Provide a list of all physical locations: include the address and phone number for each, include a main contact name for each location, and include the group or individual NPI number for each location. Please also include a contact name and email address for the EMR/EHR vendor.
4. Review the eCR Onboarding Guide.
The Onboarding Process
![]()
Partnering to Improve Health Information Technology.
Page Content Updated 05_13_2025